IPO News | Jingze Biotech submitted a total of eight drug candidates to the Hong Kong Stock Exchange for the second time
The Zhitong Finance App learned that according to the Hong Kong Stock Exchange's disclosure on December 31, Jingze Biopharmaceuticals (Hefei) Co., Ltd. - B (abbreviation: Jingze Biotech) submitted a listing application to the main board of the Hong Kong Stock Exchange, with CICC and Guoyuan International as co-sponsors. The company submitted a statement to the Hong Kong Stock Exchange on June 27, 2025.

Company profile
According to the prospectus, the company is a biopharmaceutical company with a history dating back to 2014, focusing on the two major racetracks of assisted reproductive drugs and ophthalmic drugs. The company has: (1) two core products, of which (a) JZB30 is a registered recombinant human follicle-stimulating hormone (rhFSH) injection, intended for use in (i) ovulation promotion in assisted reproductive treatment. It is suitable for adult female patients requiring controlled ovarian stimulation due to non-ovulatory infertility and (ii) treatment of infertility caused by hypogonadotropic hormone hypogonadism. It is suitable for adult male patients, and (b) JZB05 is an anti-vascular endothelial growth factor (VEGF) vitreous injection drug candidate, intended for the treatment of fundus neovascularization diseases ( FND), including wet age-related macular degeneration (wAMd), diabetic macular edema (DME), and other FND, is suitable for adult patients with active neovasal disease requiring anti-VEGF treatment; and (2) six pipeline products.
Since its establishment, the company has built a rich product pipeline to address diverse clinical needs in the field of assisted reproduction and ophthalmology. As of the last practical date (December 21, 2025), the company has eight drug candidates, three of which are in advanced stages, including (i) one drug candidate has been approved by the NDA, (ii) one drug candidate has submitted an NDA, and (iii) one product is in phase 3 clinical research. Additionally, the company has several other drug candidates in various clinical stages. The product portfolio targets clinical indications in assisted reproduction and ophthalmology.
Core products
JZB30 is the company's first commercial product in the field of assisted reproduction treatment. It is a recombinant human follicle-stimulating hormone (rhFSH) freeze-dried powder injection-type product developed by the company. It is an ovulation-promoting drug during the assisted reproduction cycle. JZB30 is a biosimilar developed as a benchmark for Gonafine®, an imported product with the highest share in the global ovulation promotion market in 2024 and the first quarter of 2025. The company has completed phase 1 and phase 3 clinical studies comparing JZB30 with the control drug (gonafine®) to receive controlled ovarian stimulation and ovulation promotion treatment with assisted reproductive technology. The NDA for JZB30 was approved by the National Drug Administration in April 2025. The company is also developing expanded indications for JZB30 for the treatment of hypogonadotropic hypogonadism.
JZB05 is expected to be the company's first commercial product in the field of ophthalmology. It is an anti-VEGF intraocular injection independently developed by the company, which is mainly used to treat FND such as WamD and DME. JZB05 is a biosimilar of the world's highest-selling ophthalmic drug and the anti-VEGF drug abacept (Aria®). According to Frost & Sullivan, Abbotsip (Aria®) achieved annual sales of US$9.5 billion in 2024. As of the last practical date, JZB05 has completed a phase 1 clinical study with head-to-head comparison with the original research drug (Aria®), and entered a phase 3 clinical study for further comparison in September 2023. The company expects to complete phase 3 clinical studies and submit an NDA listing application in the second half of 2026.
The company has patents for various inventions related to core products. As of the last practical date, the company held a total of six authorized invention patents, three pending invention patent applications, and one pending design patent application for its core products in China.
Main products
JZB33 is a recombinant human follicle-stimulating hormone injection independently developed by the company. It is also an ovulation-promoting drug for assisted reproductive cycles, and is a biosimilar drug developed in the Tegonafine® water injection form. During the ovulation promotion process, depending on the woman's follicle growth, it is usually necessary to inject 1-4 injections regularly every day for 10-14 days. Therefore, the convenience of injections is very important to ensure patient compliance. JZB33 changed the dosage form to a water needle based on JZB30, and was equipped with a pre-filled cartridge injection pen to facilitate patients to self-inject, greatly improving the convenience of medication use and covering the medication needs of different patients, thus enriching the company's product portfolio in assisted reproduction and ovulation promotion.
Relying on the clinical research foundation of JZB30, the company has obtained approval from the National Drug Administration to simplify clinical research on JZB33. By carrying out clinical trials of bioequivalence with the original drug (Gonafine®) based on pharmacokinetic parameters, plus the results of the phase 3 clinical trial of JZB30, it is possible to apply for an NDA. The company has completed the bioequivalence study for JZB33, submitted an NDA in June 2025, and has been accepted for approval.
JZB32 is a recombinant human truncated plasminase (octonolase) injection independently developed by the company to treat symptomatic vitreous macular adhesions (SvMA). Octinolase is a microfibrinolytic enzyme that optimizes structural instability based on endogenous plasminase and retains catalytic properties to break down adhesive tissue. Compared to vitrectomy, physical adhesions are removed with a microscalpel. Oak plasminase is similar to a molecular scalpel. It uses abnormal adhesive components as a substrate to complete rapid and accurate enzymatic degradation, thereby removing the adhesion or traction state of the vitreous to the retina and saving eyesight. According to Frost & Sullivan's data, JZB32 is the first Oke plasminase product under development in China. As of the last practical date, JZB32 is still in phase 1 clinical research.
In addition to SvMA, the company is exploring the application of JZB32 to other fields of fundus diseases, and is exploring the application of JZB32 in polypoid choroidal angiopathy (PCV) using Class 2.2 improved biological product research to further unlock its clinical potential.
Financial data
revenue
For the six months ended June 30 in 2023, 2024, and 2025, the company's other revenue was RMB 9.799 million, RMB 12.381 million, and RMB 829,000, respectively.
loss
For the six months ended June 30 in 2023, 2024, and 2025, the company's total losses and overall losses during the year were approximately RMB 246 million, RMB 243 million, and RMB 128 million, respectively.
R & D
For the six months ended June 30 in 2023, 2024, and 2025, the company spent approximately RMB 122 million, RMB 133 million and RMB 507.68 million respectively.
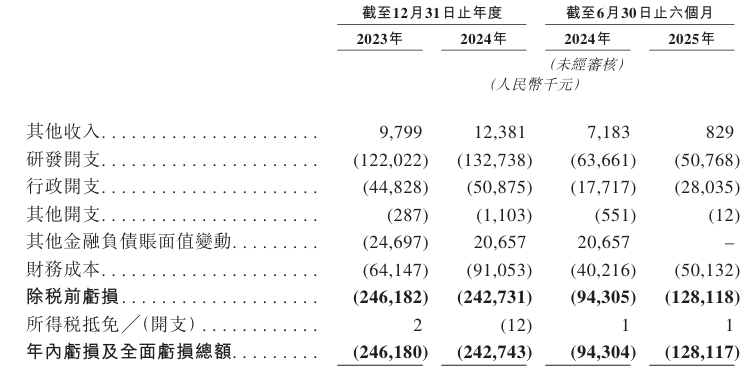
Industry Overview
Global biopharmaceutical sales increased from US$286.4 billion in 2019 to US$441.9 billion in 2024, at a CAGR of 9.1%, and are expected to grow at a CAGR of 11.0% from 2025 to 2030 to reach US$828.6 billion.
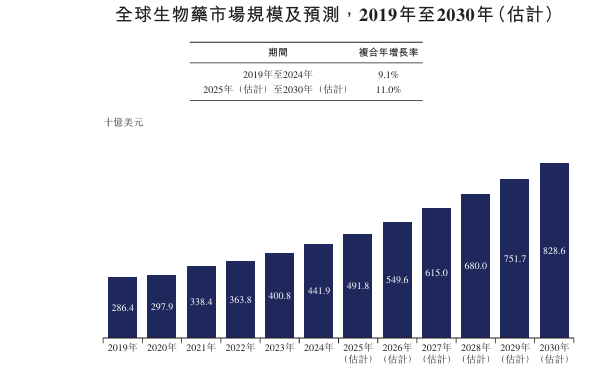
China's biopharmaceutical market grew from RMB 312 billion in 2019 to RMB 493 billion in 2024, with a compound annual growth rate of 9.6%, and is expected to grow at a compound annual growth rate of 13.1% from 2025 to 2030 to reach RMB 1,021 billion.
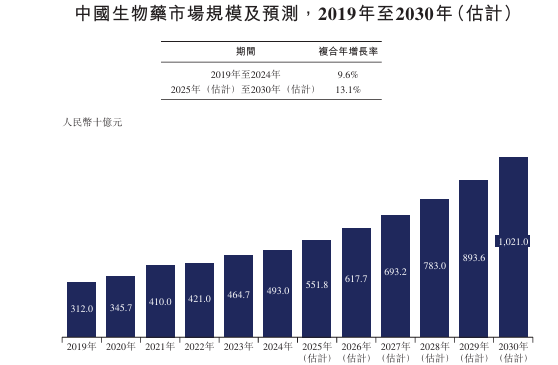
The market size of assisted reproductive drugs in China increased from RMB 4.2 billion in 2019 to RMB 5.7 billion in 2024, with a compound annual growth rate of 6.0%. It is expected to continue growing at a CAGR of 13.5% from 2025 to 2030, reaching RMB 11.9 billion.
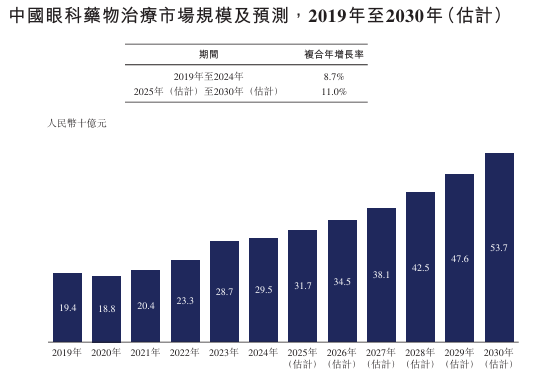
The market size of ophthalmic drugs in China grew at a CAGR of 8.7% from RMB 19.4 billion in 2019 to RMB 29.5 billion in 2024. It is expected to continue to grow to RMB 53.7 billion in 2030, with a CAGR of 11.0% from 2025 to 2030.
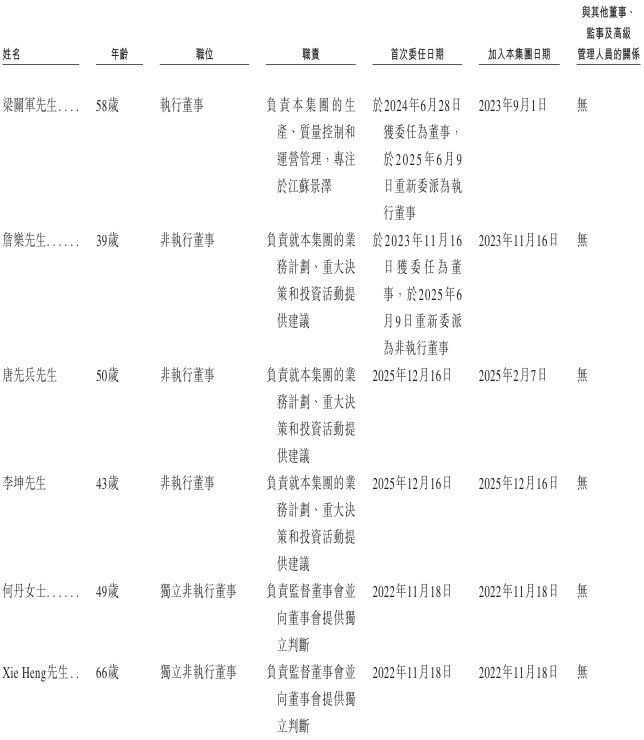
Board Information
The Board consists of nine directors, including 2 executive directors, 3 non-executive directors and 4 independent non-executive directors. The board of directors is responsible for and has general powers for the management and operation of the Group's business, including defining business strategies and investment plans, formulating group management measures, implementing resolutions passed by the Shareholders' Meeting, and exercising other powers, functions and responsibilities conferred by the Articles of Association. The Board of Directors is also responsible for formulating and reviewing the company's policies and practices relating to corporate governance, risk management and internal control, and compliance with legal and regulatory requirements.


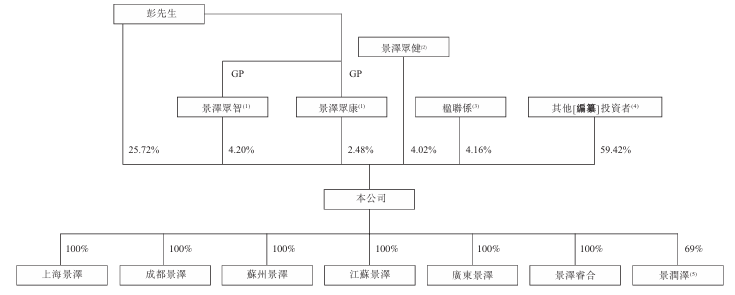
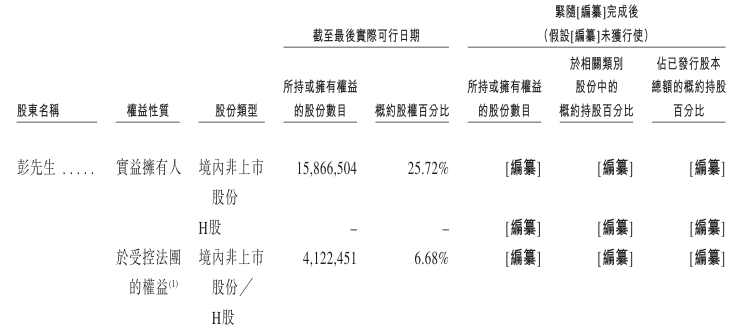
Shareholding structure
At the last practical date, Mr. Peng held approximately 32.40% of all issued shares, including about 25.72% of direct interests and about 6.68% of indirect interests held through Jingze Zhongzhi and Jingze Zhongkang (whose general partner is Mr. Peng, and Mr. Peng controls the exercise of voting rights of shares held by Jingze Zhongzhi and Jingze Zhongkang).
Intermediary team
Co-sponsors: China International Finance Hong Kong Securities Limited and Guoyuan Finance (Hong Kong) Limited;
Legal advisors: Fuld Law Firm, Tianyuan Law Firm;
Auditors and reporting accountants: Ernst & Young;
Industry consultant: Frost & Sullivan (Beijing) Consulting Co., Ltd. Shanghai Branch;
Independent Property Valuer: Beijing Colliers International Land Real Estate Asset Appraisal Co., Ltd.
 Nasdaq
Nasdaq Wall Street Journal
Wall Street Journal