Hanno Medical Science and Technology Innovation Board has accepted the core product LifeMotion® ECMO system has covered more than 140 hospitals across the country
The Zhitong Finance App learned that on December 23, Shenzhen Hanuo Medical Technology Co., Ltd. (abbreviation: Hanuo Healthcare) was accepted for the Shanghai Stock Exchange Science and Technology Innovation Board IPO. CITIC Securities is its sponsor and plans to raise 1,062 billion yuan.
According to the prospectus, Hanuo Healthcare is a high-end innovative medical device company with global competitive potential. It focuses on technological innovation in the field of in vitro life support (ECLS), and strives to provide stable and reliable circulation support and oxygenation technology products to the world with forward-looking medical engineering technology.
The company focuses on in vitro life support (ECLS), a platform-based key technology. The company's core product, Lifemotion® extracorporeal membrane pulmonary oxygenation (ECMO) system, was launched in 2023. It is the first domestic enterprise in China to successfully develop an extracorporeal membrane pulmonary oxygenation (ECMO) system and was approved for listing, achieving China's “zero breakthrough” in this field. Prior to that, only a few countries in the world, such as the US, Germany, and Italy, had the ability to industrialize this product. The company is also based on global competition. It is one of the few manufacturers in the world that has completed the complete layout of ECMO equipment and consumables at the same time. The core product, Lifemotion® ECMO system, was the first domestic ECMO system in China to enter the international market. It obtained EU CEMDR certification in early 2025, and commercialized the ECMO system domestically and overseas, fully demonstrating the level of development of the new quality productivity of Chinese medical devices, indicating that domestic high-end medical devices already have international competitiveness.
Through years of independent R&D and innovation investment, Hanno Medical has formed an interdisciplinary R&D team covering the fields of extracorporeal circulation, biomedical engineering, mechatronics, materials, precision manufacturing, and clinical medicine. It has successfully broken through many key core technologies and achieved a breakthrough in the entire domestic ECMO chain from principle to product. As of June 30, 2025, the company's core product, the Lifemotion® ECMO system, has covered more than 140 hospitals across the country, and has successfully entered the top three hospitals, including Beijing Anzhen Hospital affiliated to Capital Medical University, the Chinese People's Liberation Army General Hospital (301 Hospital), West China Hospital of Sichuan University, and Hong Kong Mary Hospital, a well-known public hospital in Hong Kong. At the beginning of 2025, the Lifemotion® ECMO system obtained EU CEMDR certification, becoming the first domestic ECMO system to enter the international market. As of the date of signing the prospectus, the company has delivered orders to Europe, South America and Africa, and has declared product registration in many countries and regions around the world.
The company adheres to clinical needs as the guide, and has mastered many key technologies such as innovative high-safety, high-stability software and hardware architecture design, artificial heart/lung design and manufacturing. The system has built (1) a high-safety and high-stability software and hardware R&D platform for extracorporeal life support auxiliary equipment; (2) a high-performance artificial heart-lung and consumables R&D and process platform; and (3) a high-performance medical coating material development and coating process platform.
Based on an independent core technology platform, the company lays out an extracorporeal circulation support system covering the full flow range, accurately matches target-oriented organ perfusion and oxygenation parameters, and provides comprehensive and flexible multi-department life support solutions. Currently, the approved product pipeline can cover the four major solutions of extracorporeal membrane pulmonary oxygenation system (ECMO), extracorporeal cardiopulmonary resuscitation system (ECPR), extracorporeal cardiopulmonary support system (CPS), and low-flow extracorporeal circulation support system (Low-Flow), extending from ultimate life support to critical care support, complex surgery, organ transplantation and innovative therapies, with broad future market prospects and commercial potential.
The successful development of the ECMO system has verified the company's technical ability to provide high-flow cardiopulmonary support. Facing diverse clinical needs and pain points with huge potential for in vitro life support, the company will use the core product Lifemotion® ECMO system as an entry point to overcome existing unmet clinical pain points by continuing to focus on gaps in vitro life support. Through core technology autonomy, supply chain diversification, and ecological collaboration, we will build industrial chain clusters, lead domestic substitution and global expansion, and promote the inclusion of high-end life support technology to the world.
This time, the company plans to publicly issue no more than 25.7515 million RMB common shares. The capital raised was reviewed and approved by the 12th meeting of the first board of directors of the company and the 3rd Extraordinary General Meeting of Shareholders in 2025. If the current stock issuance is successful, the funds raised (after deducting the issuance fee) will be invested in the following projects according to the priority of the investment project:
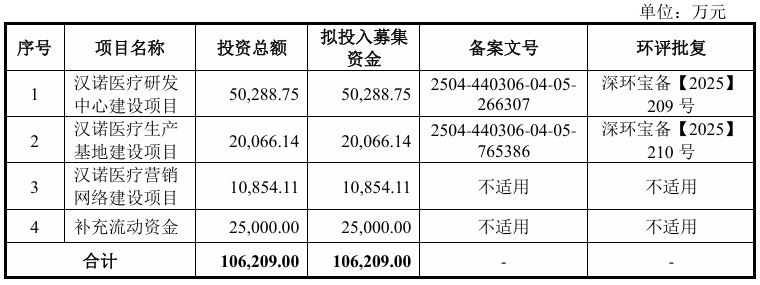
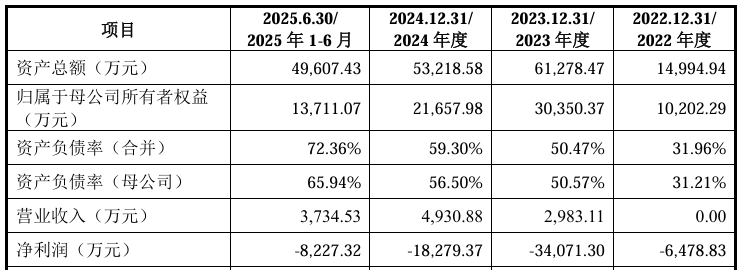
On the financial side, in 2022, 2023, 2024, and January-June 2025, the company achieved operating income of 0, 298.311 million yuan, 49,3088 million yuan, and 37.3453 million yuan respectively; in the same period, net profit was approximately -64.783 million yuan, -341 million yuan, -183 million yuan, and -82.2732 million yuan, respectively.
 Nasdaq
Nasdaq 華爾街日報
華爾街日報