Break through FAP target technology bottlenecks! Yuanda Pharmaceutical (00512) self-developed and innovated RDC US clinical was approved by the FDA to control the 100 billion oncology drug market
The Zhitong Finance App learned that leading nuclear drugs have brought huge benefits! Recently, GPN01530, a globally innovative FAP target radionuclide conjugate drug (RDC) independently developed by Yuanda Pharmaceutical (00512), was officially approved by the US FDA to conduct phase I/II clinical studies to diagnose solid tumors.
According to reports, GPN01530 is Yuanda Pharmaceutical's first self-developed RDC product approved by the FDA to carry out clinical research. This clinical approval is an important milestone in Yuanda Pharmaceuticals' “Go Global” strategy. It not only provides an important paradigm for the international development of its nuclear drug product pipeline, but also highlights the company's comprehensive strength in cutting-edge nuclear drug technology platform construction, international clinical development and registration reporting. At the same time, GPN01530 is also expected to add another important example for China's innovative drugs to go global with its best-in-class (BIC) potential.
Optimizing FAP ligand structures to solve technical bottlenecks, and pan-cancer diagnosis enhances market competitiveness
FAP (fibroblast-activating protein) is an important marker of cancer-associated fibroblasts (CAFs)). It participates in processes such as extracellular matrix remodeling, tumor cell proliferation regulation, and tumor immunosuppression to promote tumor growth and invasion, and is a novel specific target for cancer diagnosis and treatment. Studies have shown that FAP is not expressed or underexpressed in normal tissues, but is highly expressed in 90% of epithelial tumor tissues and CAFs in various tumor microenvironments. In terms of cancer diagnosis, compared with fluorine [18F] -fluoride deoxyglucose (18F-FDG), the PET/CT imaging agent currently commonly used in clinical practice, the imaging agent targeting FAP has the characteristics of being used in pan-solid tumors and has a detection sensitivity of 80% to 90% in gastric cancer, colorectal cancer, and liver cancer, which is significantly higher than the sensitivity of 18F-FDG of about 40% to 68%. The potential for clinical application of FAP targets in cancer diagnosis and treatment has become an industry consensus, and radiopharmaceutical research and development for this target is evolving from early research acceleration to clinical research.
Relying on the accumulation of R&D technology in the field of nuclear medicine, Yuanda Pharmaceutical innovatively optimized the structure of FAP ligands - the company GPN01530 increases uptake in tumor tissue while reducing its uptake in normal tissues. Preclinical study results showed that GPN01530 showed rapid tumor targeting, higher tumor uptake, and better pharmacokinetic properties compared with other FAP ligands.

Furthermore, it has been shown in IIT human studies that have been carried out that the product is safe, has rapid background removal and strong and long-lasting lesion ingestion. Compared with 18F-FDG, it shows better clinical image contrast and accurate detection rate of positive lesions. Based on existing preclinical and clinical research results, GPN01530 significantly improved the diagnostic efficacy of FAP target RDC drugs. GPN01530 is expected to break through the current technical bottleneck of FAP target RDC drugs, or provide a new tumor diagnosis solution for a large number of patients with solid tumors. It is a drug with significant BIC potential. At the same time, based on FAP targets, GPN01530 may have the potential to achieve clinical application of “pan-cancer types”, and is expected to expand to other common cancers such as gastric cancer in the future, which will also give it a stronger market competitiveness.
According to GLOBOCAN data, there are about 20 million new cancer cases and 9.7 million deaths worldwide in 2022. It is predicted that by 2030, the number of new cancer cases will be close to 24 million, and the number of deaths will be close to 12 million. In terms of market size, the global oncology drug market has grown from US$150.3 billion in 2020 to US$253.3 billion in 2024, with a compound annual growth rate of 13.9%, and is expected to grow at a compound annual growth rate of 10.2% to US$452.5 billion in 2030. The market space for cancer diagnosis and treatment drugs is huge.

In the future, if GPN01530 is successfully developed, this product may become an important breakthrough in solving the difficult problem of diagnosis and treatment of solid tumors, reshaping the pattern of solid tumor diagnosis and treatment with advantages such as high specificity and integrated diagnosis and treatment, bringing new hope to patients around the world. At the same time, Yuanda Pharmaceutical will also use this product as a basis to further promote international clinical research and registration of more self-developed and innovative products, and continue to enhance the company's global core competitiveness in the nuclear medicine field.
Go Global provides a template for sustainable overseas travel to continue to stabilize the global competitiveness of nuclear drugs
GPN01530's smooth launch into the sea coincided with the explosion of the global nuclear drug industry. According to the data, the global radiopharmaceutical market size is about 9.7 billion US dollars in 2024, and is expected to rise to 57.3 billion US dollars by 2035, with a compound annual growth rate of about 17.5%. In China, the market size is expected to grow from RMB 7.4 billion in 2024 to RMB 75.8 billion in 2035, with a CAGR of 23.5%. The radiopharmaceuticals market will continue to grow rapidly as imaging technology and radioligand therapy continue to advance and the aging of the population increases.
Yuanda Pharmaceutical's forward-looking layout in this field has formed a clear first-mover advantage. It not only has a rich and world-leading product matrix, but has also achieved a comprehensive layout of R&D, production, distribution, and sales in the industrial chain. The company has achieved a global nuclear medicine industry chain based on R&D bases in Boston and Chengdu, production bases in Boston, Frankfurt, Singapore and Chengdu, and a sales network covering more than 50 countries and regions around the world.
In terms of product pipeline, Yuanda Pharmaceutical is the company with the largest total reserves of innovative diagnostic and therapeutic RDC drugs in phase III clinical research in China. It is also one of the innovative pharmaceutical companies with the richest product pipeline and integrated diagnosis and treatment layout in the field of nuclear drugs and anti-cancer treatment in the world. At present, the company has stocked 16 innovative products in the R&D registration stage, covering 5 types of radionuclides including 68 Ga, 177 Lu, 131I, 90Y, and 89Zr, covering 7 types of cancer including liver cancer, prostate cancer, brain cancer, etc.; in the early development stage, RDC drugs are the main focus, and there are more than 10 product reserves. In terms of product types, it covers the diagnosis and treatment of two types of nuclide drugs, providing patients with a world-leading anti-tumor solution with multiple treatment options, multiple methods, and integrated diagnosis and treatment.
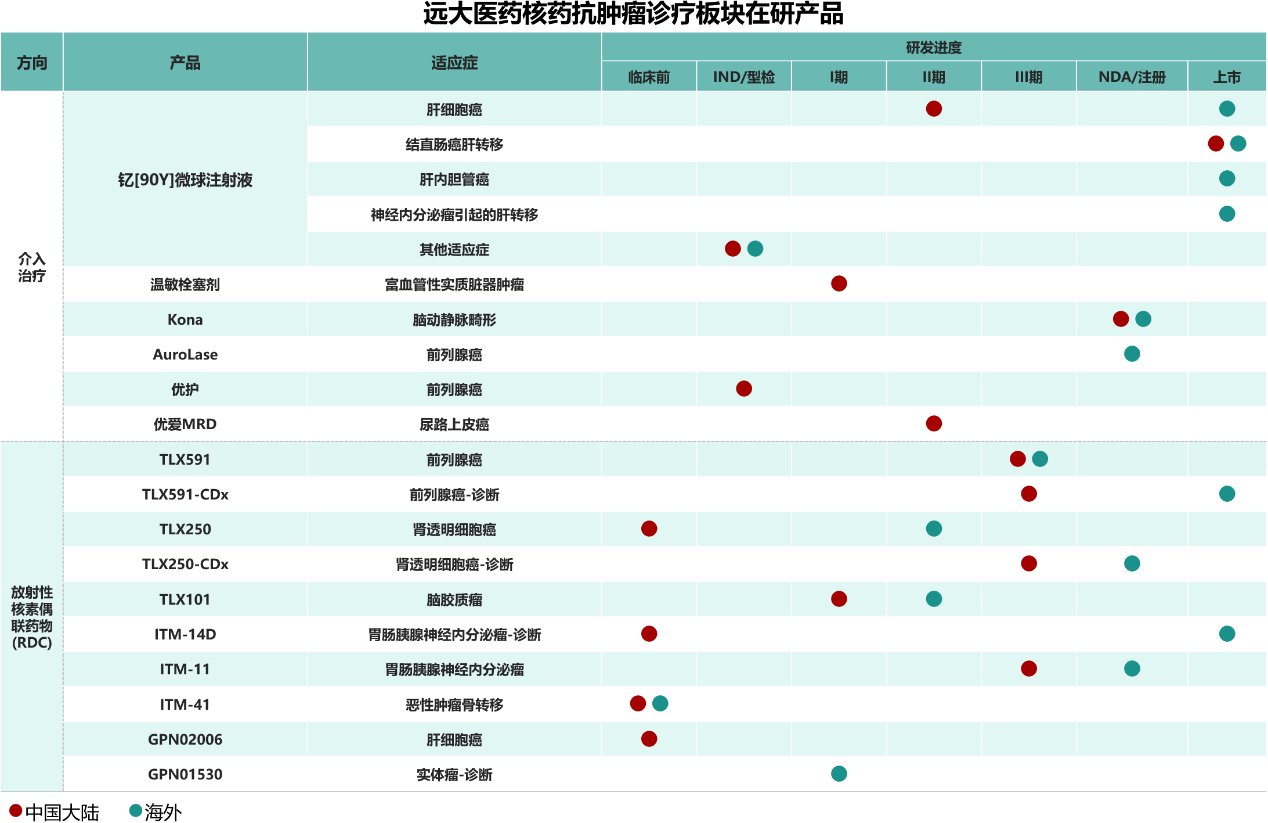
Based on the advantages of the company's forward-looking layout, Yuanda Pharmaceutical took the lead in verifying the clinical value of nuclear drug treatment products in China. In 2024, the rapid release of the company's Sir-spheres® yttrium [90Y] microsphere injection (Yigantai®) led to a year-on-year increase of over 170% in the nuclear drug anti-tumor diagnosis and treatment sector. In addition, the company's overseas nuclear drug research and development agency is also progressing smoothly, and its application for the marketing of ITM-11, an innovative RDC product for the treatment of gastrointestinal pancreatic neuroendocrine tumors (GEP-NETs), in the US was officially accepted by the FDA earlier.
In terms of industrial layout, Yuanda Pharmaceutical has built the world's first closed-loop platform for the entire nuclear drug industry chain, established a closed loop system covering the entire industry chain, and truly achieved complete autonomy and control of the entire R&D, production and sales industry chain of innovative nuclear drug products. The company's radiopharmaceutical R&D and production base in Wenjiang, Chengdu obtained a Class A “Radiation Safety License” issued by the Ministry of Ecology and Environment in May 2025 and was officially put into operation in June this year. It is currently one of the smart factories with the most complete range of nuclides and the highest degree of automation in the world, which can fully meet the diverse and large-scale preparation needs of Yuanda Pharmaceutical's therapeutic and diagnostic nuclear drugs.

It is worth noting that the early research of this GPN01530 project was based on the complete independence of the emission labeling platform and animal molecular imaging platform at Yuanda Pharmaceutical's Chengdu Nuclear Drug Base. The labeling process development and animal imaging research work were completed in one stop, and the production, inspection and release of the registered batch was achieved by relying on the GMP production line. At the same time, GPN01530 is also the first self-developed product to enter the FDA clinical phase since it was put into operation at the company's Chengdu Nuclear Drug Base in June this year, demonstrating the excellent pre-clinical development and international registration capabilities of this technology platform.
The global layout of Yuanda Pharmaceutical's nuclear medicine industry is a concentrated demonstration of the deepening of the company's “Go Global” strategy, and the implementation of the company's strategy is also reshaping the global narrative of innovative drugs in China, providing a sustainable benchmark path for domestic pharmaceutical companies. With the support of the “China and US Double News” international registration channel, Yuanda Pharmaceutical has firmly grasped the global market value of innovative drugs into its own hands and continues to consolidate the company's core competitiveness in the nuclear medicine field. In the future, with the successful development of innovative products such as GPN01530 and the continued deepening of the nuclear drug industry layout, Yuanda Pharmaceutical will also continue to advance towards MNC, the world's number one nuclear drug.
 Nasdaq
Nasdaq 華爾街日報
華爾街日報